What is pharmacovigilance?
15 December 2017
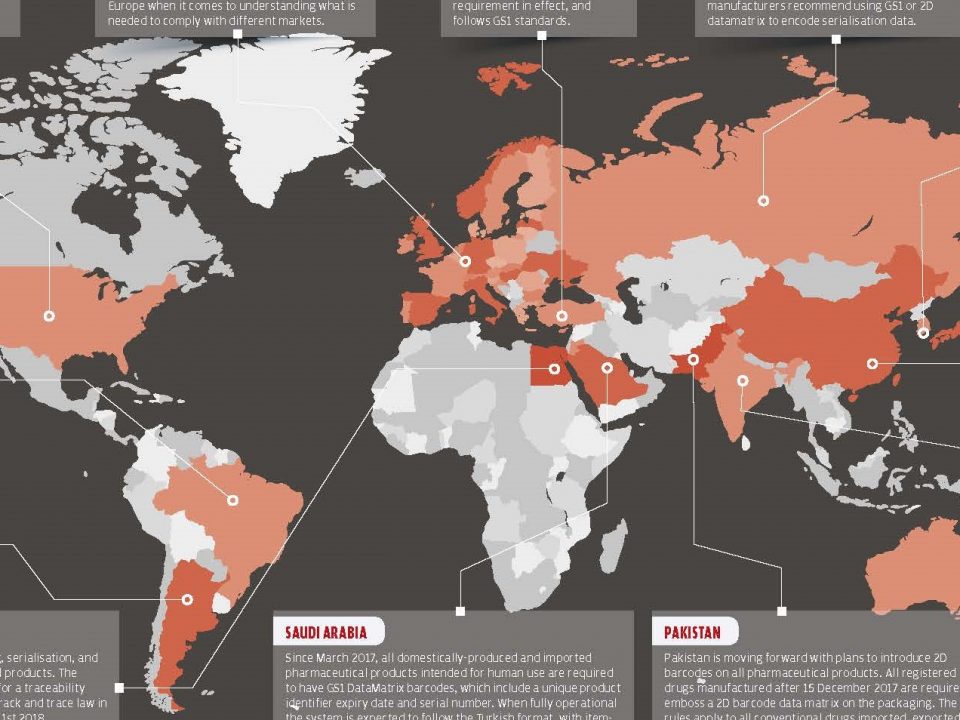
Track and Trace Obstacles, Solutions and Benefits
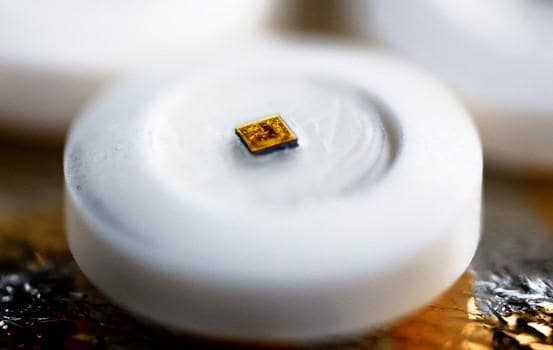
29 December 2017On 14th November 2017, U.S. Food and Drug Association approved the brand new product which is produced by Japanese Otsuka Pharmaceuticals Co. and has a pill shape and internal digital track mechanism for pysciatric treatments. The new pill is called Abilify MyCite and its active ingredient is aripiprazol which is used for the tratment of schizophrenia, bipolar and pysciatric diseases.
After the news published on 14th November 2017, the thought of new pill comes to the minds. Will it come to Turkey or not? In 2012, one of the oldest pharmaceutical company of Japan, Otsuka Pharmaceuticals Co. united with Turkish Abdi Ibrahim and named as Abdi Ibrahım Otsuka. In this matter, as Otsuka Pharmaceuticals Co. is the prodcuer company of Abilify MyCite and two companies came together, so it can be possible to bring this new technology to our country, but it is only a question mark for now.
When we have a look, the consumption of anti-depression and anti-psychotic drugs are really high. According to the analyses of Ajans Press in 2017 June, the consumpiton of anti-psychotic drugs increased from 7 M. to 12 M. and158 Thousands of boxes in last 5 years, however, it is not know how regularly these drugs are used.
With this new system developed by Otsuka Pharmaceuticals Co. it is possible to provide traceability for drug usage of patients, to report the notification of drug usage to doctors and patients’ relatives.
This system is very important in terms of traceability and it is thought to be used to regulate the drug usage of patients and to get quick responses for their treatments. Well, how does the system work? There is a digestable sensor in the pill which becomes active when it interacts with stomach fluids. On the other hand, the patients wears a patch on his or her torso. So, once he or she gets the pill the information is notified to the patch and it trasfers this data to the smartphone application. The doctor and family of the patient can get permisson to reach this data to see drug tracking through an online platform.