Solution for the Substandard and Counterfeit Medicines
14 March 2018World Pharmaceutical Market Is Growing, Oncological Pharmaceuticals Will Have A Great Share!
27 April 2018By 2020, serialization is expected to cover 80% of the global drug supply, but the regulations of different regions and increasing costs of new supply chain upgrades are making the transition process difficult and complex for the stakeholders (manufacturers, wholesalers, pharmacies etc.).
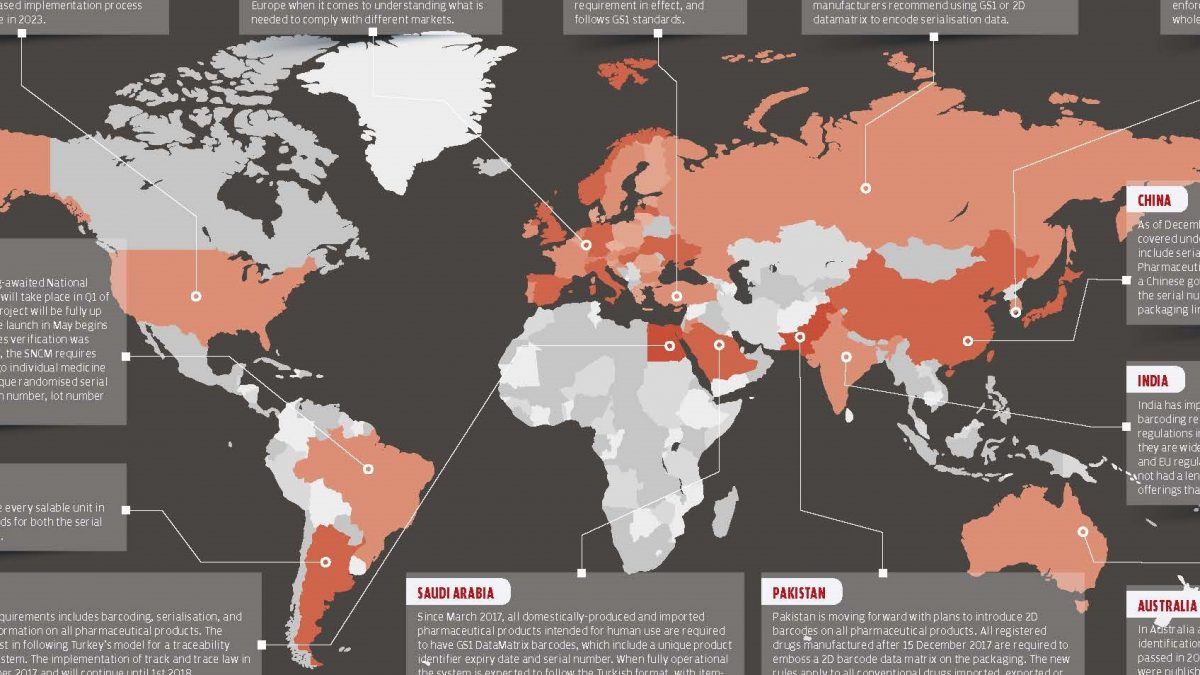
As the globally determined deadline for serialization approaches, the serialization practices of the countries have begun to settle. We will look at the 2018 World Serialization Map which prepared by Pharmaceutical Serialization & Traceability Conference Organization to see what stages of the serialization and traceability of the countries mentioned are going on.
Turkey
Since 2011, all drugs used in Turkey has been under surveillance with the Pharmaceutical Track & Trace System.
All the tracking and tracing for the medicines is in accordance with GS1 standards. With the Drug Tracking System, stages of the supply chain are monitored end to end and medicine safety is provided.
European Union
A serialisation programme typically takes six to nine months to implement according to the standarts fully. With this knowledge on hands, businesses hope to meet the February 2019 FMD deadline and to meet the deadline they should start their projects in Spring 2018. There is still considerable uncertainty within Europe when it comes to understanding what is needed to comply with different markets.
United States of America
Since November 2017, the Drug Supply Chain Safety Act (DSCSA) requires manufacturers to label packages with a product identifier, serial number, lot number, and expiration date. An assessment made six months before the deadline shows that only 6.6 percent of medicines in the US were found to have four data members DSCSA considered necessary. The DSCSA has a phased implementation process planned to be fully implemented until 2023.
Russia
Russia is currently working towards gradual implementation. From the end of 2018, this practice and the law will apply to all medicines in circulation. The prepared law does not clearly define the standards, but industry associations and producers recommend using the GS1 standard and the 2D data matrix because they are accepted all over the world.
South Korea
Since 2016, serialization practices in South Korea include all medicines in the country. Starting July 1, 2017, serialization, which is mandatory for producers, also will become mandatory for wholesalers and pharmacies.
China
Since December 2015, all pharmaceutical products have been included in the serialization and reporting to the government to meet the specific requirements of China is mandatory.
Pharmaceutical companies have to enter the medicines into a system where all the medicine records are kept. It is not possible for the medicines to enter the pharmaceutical market through the legal channels without entering serial numbers.
India
India implements the requirements of progressive serialization and barcoding but does not meet all the requirements of the US and EU regulations as the serialization regimes in India are implemented locally. The software industry in India does not have a long enough time to produce software that can meet all legal requirements.
Australia
A new barcode identification system for all medicines in Australia was set up in 2016. In September 2016, new regulations were issued for the Australian market, requiring labeling developed for all medicines, but these regulations do not apply to serialization. The law will be enforced in September 2020 and become mandatory for all supply chain items.
Saudi Arabia
Beginning March 2017, all domestic and foreign medicines produced for human use must have GS1 DataMatrix barcodes containing a unique product identifier, expiration date and serial number.
When fully integrated the system will have the Pharmaceutical Track and Trace System format used in Turkey.
Saudi Arabia’s system will also be planned and designed by TIGA Information Technologies who designed the Pharmaceutical Track and Trace System in Turkey.
Egypt
In Egypt, all pharmaceutical products are subject to barcoding, serialization and state reporting to the government. Egypt follow Turkey for the model for traceability and compliance reporting system. The tracking law was implemented on 1 September 2017 and continued until 1 January 2018.
Brazil
Pilot implementation for the National Drug Control System (SNCM), expected in Brazil for a long time, will take place in the first quarter of 2018. The database created for the project will be fully operational in May 2018 after 8 years of medicine validation. SNCM has introduced a 2D barcode insertion rule that includes a single randomize serial number, national registration number, lot number and expiration date into a single drug package.
*https://pharmaserialisation.iqpc.co.uk/