Supply Chain Integrity and Traceability
18 September 2017
Serialization in Pharmaceutical Industry
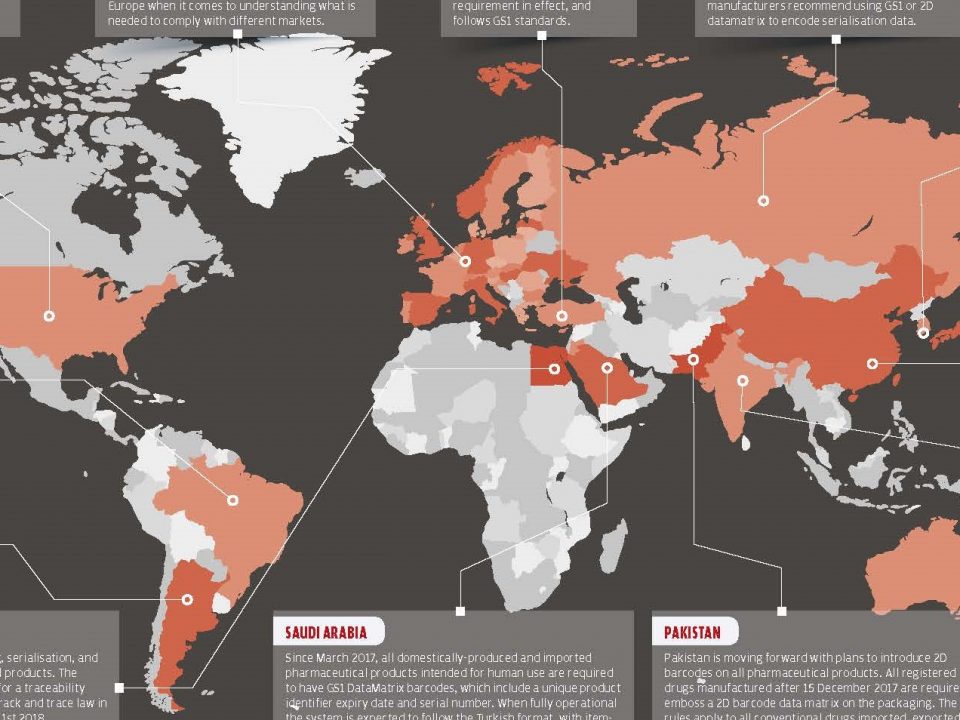
2 October 2017Counterfeit medicines are medicines that are intentionally labeled to look like the original, produced and sold in place of the original in illegal ways. These medicines may be produced from lower level or completely wrong active ingredients or may be expired and packed with new dated boxes.
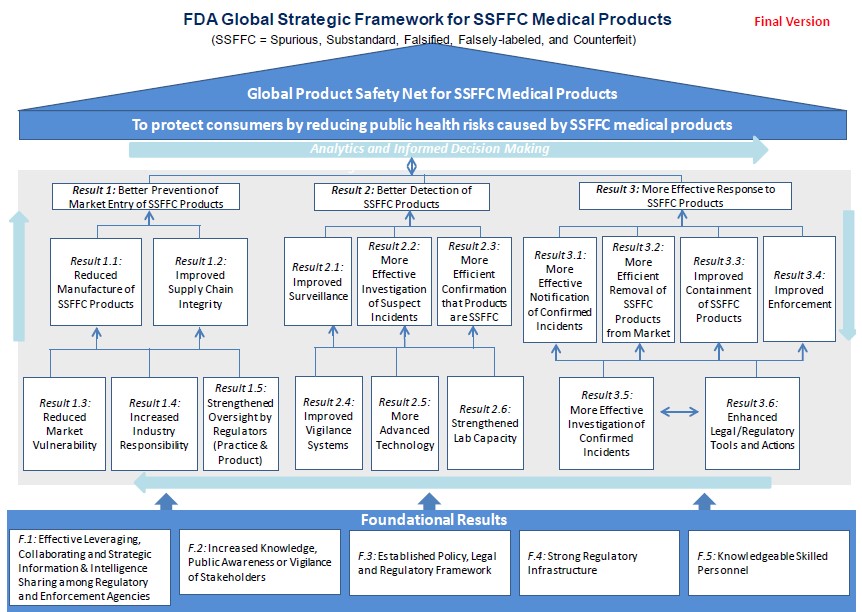
In the US, the Food and Drug Administration (FDA) has conducted a study to prevent the sale of counterfeit medicines and according to this study a plan has been prepared.*
The results expected by the FDA when the plan is implemented are as the following:
- Efficient strategic information sharing between regulatory and enforcement units
- Increase of people’s well-being
- Effective works of shareholders
- Establishment of resident policies, regulations and laws
- Preventing fake, suspicious, low quality, defective products from being produced and marketed
- Development of supply chain
- Reducing the weak points of the pharmaceutical market
- Increased use of advanced technologies and facilitation of detection of counterfeit medicines
- Establishment of advanced regulatory instruments and legal sanctions
By the end of November 2017, it became compulsory for all producers to switch to the serialized system and to adapt their procurement processes to this system. **
* https://www.fda.gov/downloads/Drugs/…/CounterfeitMedicine/UCM451841.pdf
** https://www.fda.gov/Drugs/ResourcesForYou/…/CounterfeitMedicine/